Links:
SDBS Specral Database - an awsome site where you can lookup and serach through compounds using spectral data.
UNO's Organic Compound Database - this is part of my site where you can search through organic compounds using properties.
Spectroscopy is an indespensable tool in the organic lab, in fact chemical identification of different compounds can be achived using spectroscopy alone
In CHEM 2274, the IR spectrum is evaluated before chemical tests are performed. C13 and H1 NMR spectrum is given after the lab work is done.
IR spectroscopy is the easiest to do and provides useful information on functional groups, NMR will enable you to diffrentiate between different isomers of the same compound or similar compounds.
Infrared Spectroscopy (IR)
Infrared Spectroscopy Correlation Table
Source: Wikipedia
| Bond | Type of bond | Specific type of bond | Absorption peak | Appearance |
|---|---|---|---|---|
| C=H | alkyl | methyl | 1260 cm-1 | strong |
| 1380 cm-1 | weak | |||
| 2870 cm-1 | medium to strong | |||
| 2960 cm-1 | medium to strong | |||
| methylene | 1470 cm-1 | strong | ||
| 2850 cm-1 | medium to strong | |||
| 2925 cm-1 | medium to strong | |||
| methine | 2890 cm-1 | weak | ||
| vinyl | C=CH2 | 900 cm-1 | strong | |
| 2975 cm-1 | medium | |||
| 3080 cm-1 | medium | |||
| C=CH | 3020 cm-1 | medium | ||
| monosubstituted alkenes | 900 cm-1 | strong | ||
| 990 cm-1 | strong | |||
| cis-disubstituted alkenes | 670-700 cm-1 | strong | ||
| trans-disubstituted alkenes | 965 cm-1 | strong | ||
| trisubstituted alkenes | 800-840 cm-1 | strong to medium | ||
| aromatic | benzene/sub. benzene | 3070 cm-1 | weak | |
| monosubstituted benzene | 700-750 cm-1 | strong | ||
| 690-710 cm-1 | strong | |||
| ortho-disub. benzene | 750 cm-1 | strong | ||
| meta-disub. benzene | 750-800 cm-1 | strong | ||
| 860-900 cm-1 | strong | |||
| para-disub. benzene | 800-860 cm-1 | strong | ||
| alkynes | any | 3300 cm-1 | medium | |
| aldehydes | any | 2720 cm-1 | medium | |
| 2820 cm-1 | ||||
| C=C | acyclic C=C | monosub. alkenes | 1645 cm-1 | medium |
| 1,1-disub. alkenes | 1655 cm-1 | medium | ||
| cis-1,2-disub. alkenes | 1660 cm-1 | medium | ||
| trans-1,2-disub. alkenes | 1675 cm-1 | medium | ||
| trisub., tetrasub. alkenes | 1670 cm-1 | weak | ||
| conjugated C=C | dienes | 1600 cm-1 | strong | |
| 1650 cm-1 | strong | |||
| with benzene ring | 1625 cm-1 | strong | ||
| with C=O | 1600 cm-1 | strong | ||
| C=C (both sp2) | any | 1640-1680 cm-1 | medium | |
| aromatic C=C | any | 1450 cm-1 | weak to strong (usually 3 or 4) | |
| 1500 cm-1 | ||||
| 1580 cm-1 | ||||
| 1600 cm-1 | ||||
| C=C | terminal alkynes | 2100-2140 cm-1 | weak | |
| disubst. alkynes | 2190-2260 cm-1 | very weak (often indisinguishable) | ||
| C=O | aldehyde/ketone | saturated aliph./cyclic 6-membered | 1720 cm-1 | |
| =,=-unsaturated | 1685 cm-1 | |||
| aromatic ketones | 1685 cm-1 | |||
| cyclic 5-membered | 1750 cm-1 | |||
| cyclic 4-membered | 1775 cm-1 | |||
| aldehydes | 1725 cm-1 | influence of conjugation (as with ketones) | ||
| carboxylic acids/derivates | saturated carboxylic acids | 1710 cm-1 | ||
| unsat./aromatic carb. acids | 1680-1690 cm-1 | |||
| esters and lactones | 1735 cm-1 | influenced by conjugation and ring size (as with ketones) | ||
| anhydrides | 1760 cm-1 | |||
| 1820 cm-1 | ||||
| acyl halides | 1800 cm-1 | |||
| amides | 1650 cm-1 | associated amides | ||
| carboxylates (salts) | 1550-1610 cm-1 | |||
| amino acid zwitterions | 1550-1610 cm-1 | |||
| O=H | alcohols, phenols | low concentration | 3610-3670 cm-1 | |
| high concentration | 3200-3400 cm-1 | broad | ||
| carboxylic acids | low concentration | 3500-3560 cm-1 | ||
| high concentration | 3000 cm-1 | broad | ||
| N=H | primary amines | any | 3400-3500 cm-1 | strong |
| 1560-1640 cm-1 | strong | |||
| secondary amines | any | >3000 cm-1 | weak to medium | |
| ammonium ions | any | 2400-3200 cm-1 | multiple broad peaks | |
| C=O | alcohols | primary | 1040-1060 cm-1 | strong, broad |
| secondary | ~1100 cm-1 | strong | ||
| tertiary | 1150-1200 cm-1 | medium | ||
| phenols | any | 1200 cm-1 | ||
| ethers | aliphatic | 1120 cm-1 | ||
| aromatic | 1220-1260 cm-1 | |||
| carboxylic acids | any | 1250-1300 cm-1 | ||
| esters | any | 1100-1300 cm-1 | two bands (distinct from ketones, which do not possess a C=O bond) | |
| C=N | aliphatic amines | any | 1020-1220 cm-1 | often overlapped |
| C=N | any | 1615-1700 cm-1 | similar conjugation effects to C=O | |
| C=N (nitriles) | unconjugated | 2250 cm-1 | medium | |
| conjugated | 2230 cm-1 | medium | ||
| R=N=C (isocyanides) | any | 2165-2110 cm-1 | ||
| R=N=C=S | any | 2140-1990 cm-1 | ||
| C=X | fluoroalkanes | ordinary | 1000-1100 cm-1 | |
| trifluromethyl | 1100-1200 cm-1 | two strong, broad bands | ||
| chloroalkanes | any | 540-760 cm-1 | weak to medium | |
| bromoalkanes | any | 500-600 cm-1 | medium to strong | |
| iodoalkanes | any | 500 cm-1 | medium to strong | |
| N=O | nitro compounds | aliphatic | 1540 cm-1 | stronger |
| 1380 cm-1 | weaker | |||
| aromatic | 1520, 1350 cm-1 | lower if conjugated |
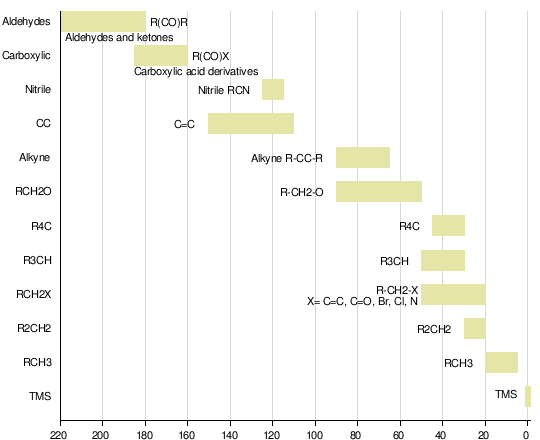
C13 NMR Spectroscopy
Carbon 13 Nuclear Magnetic Resonance Spectroscopy (C13 NMR)
Source:Wikipedia
H1 NMR Spectroscopy
Hydrogen (proton) Nuclear Magnetic Resonance Spectroscopy (H1 NMR)
Source: Wikipedia
| Functional group | CH3 | CH2 | CH |
|---|---|---|---|
| CH2R | 0.8 | 1.3 | 1.6 |
| C=C | 1.6 | 2.0 | 2.6 |
| C?C | 1.7 | 2.2 | 2.8 |
| C6H5 | 2.3 | 2.6 | 2.9 |
| F | 4.3 | 4.4 | 4.8 |
| Cl | 3.0 | 3.4 | 4.0 |
| Br | 2.7 | 3.4 | 4.1 |
| I | 2.2 | 3.2 | 4.2 |
| OH | 3.3 | 3.5 | 3.8 |
| OR | 3.3 | 3.4 | 3.7 |
| OC6H5 | 3.8 | 4.0 | 4.3 |
| OCOR | 3.6 | 4.1 | 5.0 |
| OCOC6H5 | 3.9 | 4.2 | 5.1 |
| OCOCF3 | 4.0 | 4.4 | / |
| CHO | 2.2 | 2.4 | 2.5 |
| COR | 2.1 | 2.2 | 2.6 |
| COOH | 2.1 | 2.3 | 2.6 |
| COOR | 2.0 | 2.3 | 2.5 |
| CONR2 | 2.0 | 2.1 | 2.4 |
| CN | 2.1 | 2.5 | 3.0 |
| NH2 | 2.5 | 2.7 | 3.0 |
| NR2 | 2.2 | 2.4 | 2.8 |
| NRC6H5 | 2.6 | 3.0 | 3.6 |
| NR3+ | 3.0 | 3.1 | 3.6 |
| NHCOR | 2.9 | 3.3 | 3.7 |
| NO2 | 4.1 | 4.2 | 4.4 |
| SR | 2.1 | 2.5 | 3.1 |
| SOR | 2.6 | 3.1 | / |
| =O (aliphatic aldehyde) | / | / | 9.5 |
| =O (aromatic aldehyde) | / | / | 10 |
| M-H (metal hydride) | / | / |